Chapter 11 : Aquatic and Terrestrial Plants
11.1 Introduction
11.2 Structure Provided by Plants
11.3 Influence of Plants on Fish Spawning
11.4 Aquatic Plant Establishment
11.4.1 Plant Selection
11.4.2 Source of Propagule
11.4.3 Propagule Production
11.4.4 Plant Establishment in Reservoirs
11.4.5 Multiple Depths Planting
11.4.6 Post-Planting Monitoring
11.5 Control of Aquatic Plant Growth
11.5.1 Biological Control
11.5.2 Mechanical and Physical Control
11.5.3 Chemical Control Practices
11.5.4 Cultural Control Practices
11.6 Promotion of Terrestrial Plants on Barren Shorelines
11.6.1 Wee-Vegetated Riparian Zones
11.6.2 Seeding
11.6.3 Timing
11.6.4 Seeding Methods
11.6.5 Transplanting
11.6.6 Grasses and Other Herbaceous Plants
11.6.7 Trees and Shrubs
11.1 Introduction
Plants are an important part of healthy, diverse aquatic ecosystems. Specific roles of aquatic plants and terrestrial plants that colonize reservoirs include producing and consuming of oxygen, stabilizing temperature and light, recycling nutrients, controlling turbidity, and providing food, spawning substrate, and habitat for invertebrates and fish. Plants also protect shorelines from erosion, and plant roots stabilize lake-bottom sediment to protect it from the stirring effect of wave action. Additionally, plants are valued for their aesthetic qualities and help provide a more “natural” buffer between the riparian zone and the open water.
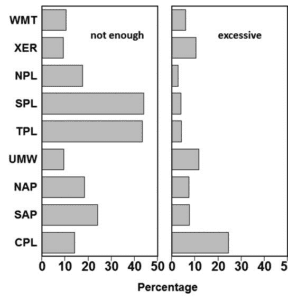
1.3. Regions include Xeric (XER), Western Mountains (WMT), Northern Plains (NPL), Temperate Plains (TPL), Southern Plains (SPL), Upper Midwest (UMW), Coastal Plains (CPL), Southern Appalachian (SAP), and Northern Appalachian (NAP).
Aquatic macrophyte abundance in reservoirs often exhibits two contrasting problems: too many or too few macrophytes. A survey of 1,299 reservoirs ≥250 ac in the USA identified that excessive macrophytes was a concern in nearly 10% of the reservoirs surveyed, and not having enough macrophytes was a concern in over 25% of the reservoirs (Krogman and Miranda 2016). These percentages varied regionally, with excessive macrophytes afflicting nearly 25% of reservoirs in the Coastal Plains ecoregion, and not enough macrophytes troubling over 40% of reservoirs in the Southern Plains and Temperate Plains ecoregions (Figure 11.1).
Three factors commonly preclude development of adequate aquatic plant densities in reservoirs. First, aquatic plant communities may take hundreds or even thousands of years to develop in natural lakes (Doyle and Smart 1993). Because most reservoirs are <100 years old, there has not been enough time to allow the development of a seed bank that can support suitable plant assemblages. Moreover, suitable seed banks may not exist in the reservoir’s watershed. Second, the abiotic conditions in many reservoirs may be too harsh for many aquatic plants. These include high turbidity and large and rapid water-level fluctuations. Third, herbivores, including various fish species, reptiles and amphibians, mammals, birds, crayfish, and insects can prevent the survival of pioneer aquatic plant colonies that eventually may colonize the reservoir.
Macrophytes occasionally can become a nuisance, but how much is too much depends on the reservoir and its use. Some uses of a reservoir are more affected by macrophytes than others, and some types of plants interfere with boating or recreational activities more than others. Generally, plants are not considered to be a problem unless they interfere with desired uses for the reservoir. Some plants have capabilities to become very abundant and are thus apt to become a nuisance. An example is the nonnative hydrilla (Hydrilla verticillata), which is found in a wide range of environments. This plant has a broad tolerance in its environmental requirements and is capable of flourishing under what seems to be difficult conditions. Recreational boaters unwittingly contribute to the spread of hydrilla and other macrophytes by carrying fragments of the plant on their boats, trailers, or fishing gear to other water bodies.
Various problems frequently are attributed to the excessive growth of macrophytes in reservoirs. Oxygen deficiencies due to plant respiration and to decay of deceased plants often are identified as a major problem for various water uses. Excessive protection of prey fish to the extent that normal predator–prey interactions are substantially diminished and alter population dynamics, fish assemblage composition, and possibly fish production are major fishery concerns. Another common complaint is the interference with recreational activities such as boating, water skiing, swimming, and bank angling. Additionally, unsightly and odoriferous accumulations of plant material can develop on the water surface, on beaches, and along property fronts.
Terrestrial plants in regulated zones of reservoirs can provide important habitat to spawning adult fish and juveniles. The regulated zone often turns into bare shorelines or mudflats because of the die-off of flood-intolerant plants, which is caused by annual or semi-annual flooding, wave action, or both (section 7). Some reservoirs, particularly in the West, have steep, bare banks with 100-250-ft drawdowns. Conversely, shallow reservoirs with smaller drawdowns can expose extensive areas encompassing hundreds or thousands of acres and representing a large fraction of the reservoir. These large areas of bare mudflats exposed during drawdowns may be recolonized by terrestrial plants during drought years when water levels remain low but otherwise remain mostly bare and provide low-quality habitat.
Back to the Top
11.2 Structure Provided by Plants
Aquatic vegetation increases the habitat complexity of reservoir ecosystems. An overabundance of plants, however, can interfere with fish feeding. In waters with no aquatic macrophytes, there may be insufficient cover to allow survival of structure- oriented small fish. As vegetation increases to intermediate levels, habitat becomes more complex, invertebrate densities increase, small prey and young predator fish find more refuge from predators, and recruitment into older age groups increases (Dibble et al. 1997; Miranda and Pugh 1997). At high levels of vegetation, especially dense monocultures formed by invasive aquatic species, it is more difficult for fish predators to forage because of the visual barrier or inaccessibility. This lack of access to prey causes overall slower fish growth, favoring small-size fish and reducing the larger fish that commonly make up a fishery. Fish assemblage composition may also shift. Reservoirs with low vegetation densities tend to include a higher abundance of fish species adapted to open-water habitats, whereas reservoirs with a high abundance of aquatic vegetation tend to be dominated by fish species adapted to cover (Bettoli et al. 1993). In addition, many fish that live among aquatic plants are visual feeders, and the shade produced by overhanging leaves and plant canopies improves visual acuity so fish can find prey and avoid becoming prey (Helfman 1981).
Researchers have suggested that a moderate amount of vegetation is optimal for fish production. Vegetation coverage of 20%–80% encourages the formation of stable fish assemblages, and 20%–40% has been reported as optimal (Durocher et al. 1984; Wiley et al. 1984; Miranda and Pugh 1997). This is a relatively wide range, which meets diverse goals of management including maintaining adequate fish and wildlife habitat.
Back to the Top
11.3 Influence of Plants on Fish Spawning

The structure provided by aquatic plants provides important habitat for fish reproduction (Petr 2000). Many fish are obligate plant spawners, directly or indirectly requiring aquatic plants to reproduce. Various fish families use vegetation as nurseries for their young, and reproductive success of nest spawners is improved when they have access to sites with aquatic vegetation, other forms of structure, or both. Fish can derive a number of benefits from nesting near aquatic plants. For example, vegetation can protect nest sites from wave action and sedimentation, which can harm eggs and larvae. Also, parents often use aquatic plant patches or edges as backing to protect nests from predators (Figure 11.2).
Back to the Top
11.4 Aquatic Plant Establishment
Become a Contributing Sponsor
Become a part of projects that need your support.

